Regulatory & Industry Standards
Ensuring Quality, Safety, and Compliance in Healthcare
Maintain the Standandards to Ensure the Excellence
At vertex medical we ensure that our products and services should meet the international and national standards.
To maintain the safety, quality and effectiveness of our products and services, we follow the national and international regulatory standards.
Our Commitment to Regulatory Compliance
At national level we are certified from all quality assuring bodies such as DRAP and SECP.
- We are the DRAP (Drug Regulatory Authority of Pakistan) registered authorised distributors of different products.
- Vertex Medical is the SECP (Securities & Exchange Commission of Pakistan) registered company.
At international level we comply with the standards of ISO, CE, WHO and FDA.
- ISO 13485 & ISO 9001 & ISO 14001 & ISO 45001 certified for managing quality of medical equipment
- CE (Conformité Européenne) Marking: It indicates that our products complies with relevant European Union (EU) laws and standards related to safety, health, and environmental protection.
- WHO (World Health Organization): We ensure the implementation of global healthcare safety and performance standards.
From legal perspective we are the authorized distributors of medical equipments in Pakistan and hold licenses and registration certificates such as
- Import & Distribution Licenses
- Healthcare & Medical Device Registration
- Regulatory standards ensure the safety and efficacy of the process and provide legal and financial security to the bussiness.
- These standards ensure safety and reduce the risk for consumers.
- A streamline regulatory process reduces the chances of error and improves productivity.
Certifications & Approvals
At Vertex Medical, we ensure that all our medical equipment, devices, and healthcare solutions come with the required certifications to ensure safety, performance, and regulatory compliance.
- ISO 9001: Quality management system for continuous improvement.
- ISO 13485: International standard for medical devices.
- ISO 14001: An international standard that provides a framework for organizations to develop an effective Environmental Management System (EMS), aiming to reduce environmental impact and promote sustainable practices.
- ISO 45001: A global standard for Occupational Health and Safety Management Systems (OHSMS), designed to help organizations proactively improve employee safety, reduce workplace risks, and create better, safer working conditions.
- DRAP Registered Products – Fully compliant with Pakistan’s
- Drug Regulatory Authority requirements.
Why Regulatory Compliance Matters in Healthcare?
- Patient Safety: Reducing risks associated with faulty or substandard equipment.
- Legal Protection: Compliance with government healthcare laws and medical ethics.
- Operational Excellence: Ensuring smooth functionality of healthcare institutions.
- Innovation & Advancement: Bringing the latest medical technologies with regulatory validation.

Quality Control & Testing Procedures
- Manufacturing Inspections: Ensuring that products meet global manufacturing standards.
- Functionality & Performance Testing: Evaluating real-world performance before market launch.
- Sterilization & Safety Procedures: Ensuring that surgical and medical devices are 100% safe for patients.
- Post-Market Surveillance: Continuous product monitoring and feedback system.
Regulatory Support & Assistance for Healthcare Providers
- Regulatory Consultation: Helping hospitals and clinics meet Pakistan’s healthcare regulations.
- Training & Awareness Programs: Educating doctors, biomedical engineers, and healthcare professionals on safe equipment usage & compliance requirements.
- Regular Compliance Updates: Keeping our customers informed about latest industry regulations and legal changes.


ISO 14001:2015

ISO 9001:2015

ISO 13485 - VERTEX MEDICAL
DSL NEW 24.03.2028


ESTABLSHMENT LICENSE


PNRA License.


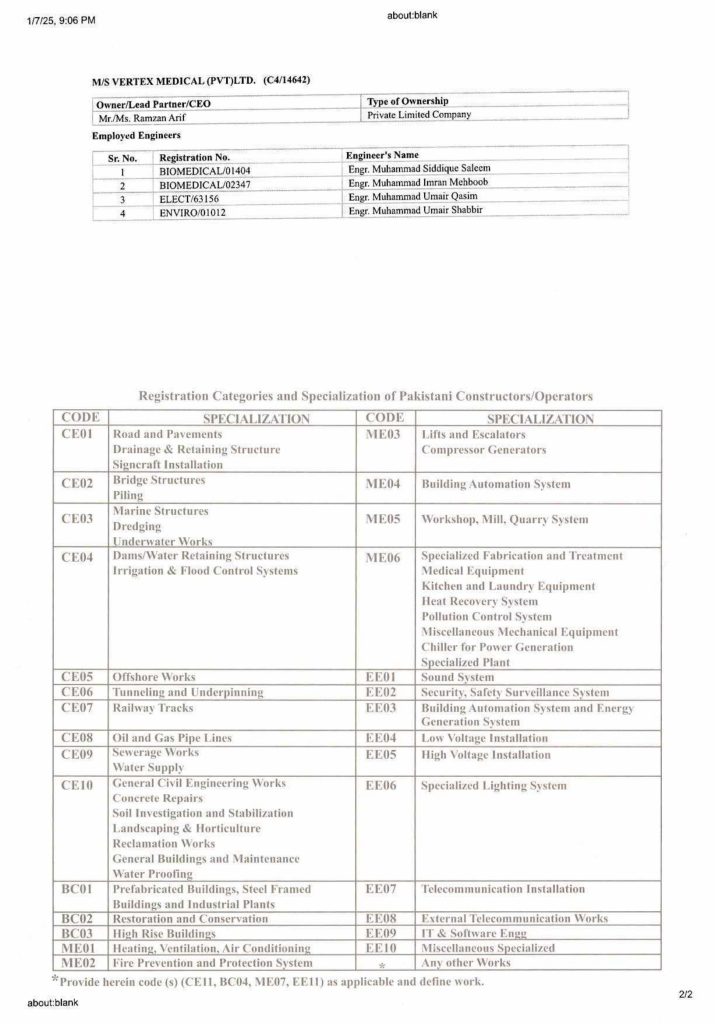
PEC Registration 30-06-2025